Harrow Announces Cash-Pay Direct-to-Prescriber PharmaPack™ Kits
PharmaPack Program to Expand Access to Affordable, FDA-Approved Branded Ophthalmic Therapies
NASHVILLE, Tenn., Feb. 17, 2026 (GLOBE NEWSWIRE) -- Harrow (Nasdaq: HROW), a leading provider of ophthalmic disease management solutions in North America, today announced the launch of a new Direct-to-Prescriber (DTP) cash-pay offering called PharmaPack, which expands the Company’s commitment to offering affordable FDA-approved branded products as alternatives to off-label compounded formulations.
PharmaPack kits expand access to FDA-approved ophthalmic products for the millions of Americans each year who require infection control and treatment of pain and inflammation associated with cataract surgery. PharmaPack kits also remove insurance-related administrative complexity and reduce regulatory and medical-legal risk associated with off-label compounded products. Harrow will offer the following PharmaPack kits at affordable and 100% transparent pricing:
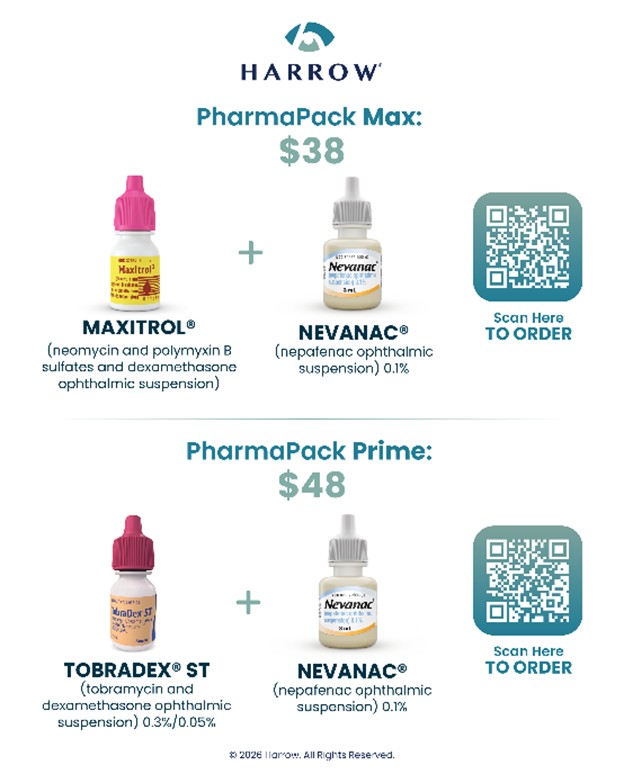
“Harrow’s PharmaPack program addresses a long-standing challenge in ophthalmology—providing patients with FDA-approved therapies that are both reliable and affordable,” said Scott Spector, MD. “The combination of trusted branded products, pricing transparency, and these compelling cash-pay prices is encouraging and supports complexity-free prescribing and greater confidence when considering FDA-approved branded alternatives to compounded products. Given the strength of this offering and the value it represents, I have already ordered PharmaPack Prime for my patients.”
“Access to FDA-approved therapies at affordable, transparent prices is something optometrists have been asking for,” said Maria Pribis, OD. “PharmaPack helps close that gap by making high-quality branded products more attainable for everyday patient care.”
How to Order:
PharmaPack kits will initially be available in California, Mississippi, Arkansas, Connecticut, and Alabama, with plans to expand nationwide in the coming weeks. Prescribers can access PharmaPack offerings from the home screen of their account dashboard, or through the following links:
Contact your Harrow or ImprimisRx representative with any questions, or email PharmaPack@HarrowInc.com.
About NEVANAC®
INDICATION AND USAGE
NEVANAC ophthalmic suspension is a nonsteroidal, anti-inflammatory prodrug indicated for the treatment of pain and inflammation associated with cataract surgery.
CONTRAINDICATIONS
Hypersensitivity to any of the ingredients in the formula or to other NSAIDS.
WARNINGS & PRECAUTIONS
- Increased Bleeding Time: With some nonsteroidal anti-inflammatory drugs including NEVANAC®, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery. It is recommended that NEVANAC® ophthalmic suspension be used with caution in patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
- Delayed Healing: Topical nonsteroidal anti-inflammatory drugs (NSAIDs) including NEVANAC®, may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
-
Corneal Effects: Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs including NEVANAC® and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 1 day prior to surgery or use beyond 14 days post surgery may increase patient risk and severity of corneal adverse events.
-
Contact Lens Wear: NEVANAC should not be administered while using contact lenses.
ADVERSE REACTIONS
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Please see additional Important Safety Information throughout and the enclosed full Prescribing Information.
About TOBRADEX® ST
INDICATION AND USAGE
TOBRADEX ST (tobramycin and dexamethasone ophthalmic suspension) 0.3%/0.05% is a topical antibiotic and corticosteroid combination for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
Important Safety Information
CONTRAINDICATIONS:
Most viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and also in mycobacterial infection of the eye and fungal disease of ocular structures. Hypersensitivity to any components of the medication.
WARNINGS & PRECAUTIONS:
- Intraocular Pressure (IOP) Increase—Prolonged use may result in glaucoma with damage to the optic nerve, and defects in visual acuity and fields of vision. IOP should be monitored.
- Aminoglycoside Sensitivity—Sensitivity to topically applied aminoglycosides may occur.
- Cataracts—Posterior subcapsular cataract formation may occur.
- Delayed Healing—May delay healing and increase the incidence of bleb formation. Perforations of the cornea or sclera have occurred. Slit lamp biomicroscopy and fluorescein staining should be conducted.
- Bacterial Infections—May suppress host response and increase secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, then the patient should be re-evaluated.
- Viral Infections—Use with a history of herpes simplex requires great caution. The course and severity of many viral infections of the eye (including herpes simplex) may be exacerbated.
- Fungal Infections—Fungal infections of the cornea may occur and should be considered in any persistent corneal ulceration.
- Vision Blurred—Vision may be temporarily blurred following dosing with TOBRADEX® ST. Care should be exercised in operating machinery or driving a motor vehicle.
- Risk of Contamination—Do not touch the dropper tip of the bottle to any surface, as this may contaminate the contents.
- Contact Lens Use—TOBRADEX® ST contains benzalkonium chloride, an antimicrobial preservative, which may be absorbed by soft contact lenses. Contact lenses should not be worn during the use of TOBRADEX® ST.
ADVERSE REACTIONS:
- The most frequent adverse reactions (<4%) to topical ocular tobramycin are hypersensitivity and localized ocular toxicity, including eye pain, eyelid pruritus, eyelid edema, and conjunctival hyperemia.
- The reactions due to the steroid component are increased IOP with possible damage to optic nerve and development of glaucoma, subcapsular cataract, and impaired healing.
- The development of secondary infection has occurred. Fungal infections of the cornea may occur. Secondary bacterial ocular infection following suppression of host responses also occurs.
- Non-ocular adverse events (0.5% to 1%) included headache and increased blood pressure.
The following additional adverse reactions have been reported with the individual components below:
- Aminoglycosides: Neurotoxicity, ototoxicity, and nephrotoxicity have occurred in patients receiving systemic aminoglycoside therapy. Aminoglycosides may aggravate muscle weakness in patients with known or suspected neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, because of their potential effect on neuromuscular function.
- Dexamethasone: Cushing’s syndrome and adrenal suppression may occur after the use of dexamethasone in excess of the listed dosing instructions in predisposed patients, including children and patients treated with CYP3A4 inhibitors.
Please see the full Prescribing Information.
About MAXITROL®
INDICATIONS AND USAGE:
- For steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where bacterial infection or a risk of bacterial ocular infection exists.
- Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe where the inherent risk of steroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns; or penetration of foreign bodies.
- The use of a combination drug with an anti-infective component is indicated where the risk of infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
- The particular anti-infective drug in this product is active against the following common bacterial eye pathogens: Staphylococcus aureus, Escherichia coli, Haemophilus influenzae, Klebsiella/Enterobacter species, Neisseria species, and Pseudomonas aeruginosa.
-
This product does not provide adequate coverage against: Serratia marcescens and Streptococci, including Streptococcus pneumoniae.
CONTRAINDICATIONS:
Maxitrol is contraindicated in patients with epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and many other viral infections of the cornea and conjunctiva, mycobacterial infections of the eye, fungal diseases of ocular structures, as well as hypersensitivity to any component of the medication.
WARNINGS:
- Maxitrol should not be injected or intraocularly administered.
- Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye. Prolonged use may result in glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infection.
- If this product is used for 10 days or longer, intraocular pressure should be routinely monitored.
- Neomycin sulfate may cause cutaneous sensitization. Sensitivity to topically administered aminoglycosides, such as neomycin, may occur in some patients. If hypersensitivity develops, treatment should be discontinued. Cross hypersensitivity to other aminoglycosides can occur, and the possibility that patients who become sensitized to topical neomycin may also be sensitive to other topical and/or systemic aminoglycosides should be considered.
PRECAUTIONS:
If inflammation or pain persists longer than 48 hours or becomes aggravated, the patient should discontinue use and consult a physician. Patients should be advised that their vision may be temporarily blurred following dosing with Maxitrol.
ADVERSE REACTIONS:
Reactions occurring most often from the presence of the anti-infective ingredient are allergic sensitizations. The reactions due to the steroid component are: elevation of intraocular pressure (IOP) with possible development of glaucoma, and infrequent optic nerve damage; posterior subcapsular cataract formation; and delayed wound healing.
To view the Full Prescribing Information for Maxitrol, please visit maxitrolrx.com/prescribinginformation.
About Harrow
Harrow, Inc. (Nasdaq: HROW) is a leading provider of ophthalmic disease management solutions in North America, offering a comprehensive portfolio of products that address conditions affecting both the front and back of the eye, such as dry eye disease, wet (or neovascular) age-related macular degeneration, cataracts, refractive errors, glaucoma and a range of other ocular surface conditions and retina diseases. Harrow was founded with a commitment to deliver safe, effective, accessible, and affordable medications that enhance patient compliance and improve clinical outcomes. For more information about Harrow, please visit harrow.com and connect with us on LinkedIn.
Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this release that are not historical facts may be considered “forward-looking statements.” Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties, which may cause results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties that could cause actual results to differ from those predicted include, among others, risks related to: liquidity or results of operations; our ability to successfully implement our business plan, develop and commercialize our products, product candidates and proprietary formulations in a timely manner or at all, identify and acquire additional products, manage our pharmacy operations, service our debt, obtain financing necessary to operate our business, recruit and retain qualified personnel, manage any growth we may experience and successfully realize the benefits of our previous acquisitions and any other acquisitions and collaborative arrangements we may pursue; competition from pharmaceutical companies, outsourcing facilities and pharmacies; general economic and business conditions, including inflation and supply chain challenges; regulatory and legal risks, including litigation matters, and other uncertainties related to our pharmacy operations and the pharmacy and pharmaceutical business in general; physician interest in and market acceptance of our current and any future formulations and compounding pharmacies generally. These and additional risks and uncertainties are more fully described in Harrow’s filings with the Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended December 31, 2024, subsequent Quarterly Reports on Form 10-Q, and other filings with the SEC. Such documents may be read free of charge on the SEC’s website at sec.gov. Undue reliance should not be placed on forward-looking statements, which speak only as of the date they are made. Except as required by law, Harrow undertakes no obligation to update any forward-looking statements to reflect new information, events, or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.
Contacts:
Mike Biega
Vice President of Investor Relations and Communications
mbiega@harrowinc.com
617-913-8890
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/65f1b48d-ca95-4242-a307-f68011e16b38

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.


